Croyez GMP ® TGF beta 1 (Transforming growth factor beta 1), Human
MALDTNYCFSSTEKNCCVRQLYIDFRKDLGWKWIHEPKGYHANFCLGPCPYIWSLDTQYSKVLALYNQHNPGASAAPCCVPQALEPLP
IVYYVGRKPKVEQLSNMIVRSCKCS with polyhistidine tag at the C-terminus
UnitProt ID
P01137
Species of Origin
Human
Expression System
Escherichia coli
Endotoxin level
<0.05 EU per 1 μg of the protein by the LAL method.
Activity
Measure by its ability to inhibit the IL-4 dependent proliferation in TF-1 cells. The ED50 for this effect is <0.1 ng/mL.
The specific activity of recombinant human TGF beta 1 is approximately >1 x 10⁷ IU/mg, which is calibrated against the human TGF beta 1 WHO International Standard (NIBSC code: 89/514).
Measure by its ability to induce proliferation in MCF-7 cells The ED50 for this effect is <3.2 ng/mL.
Purity
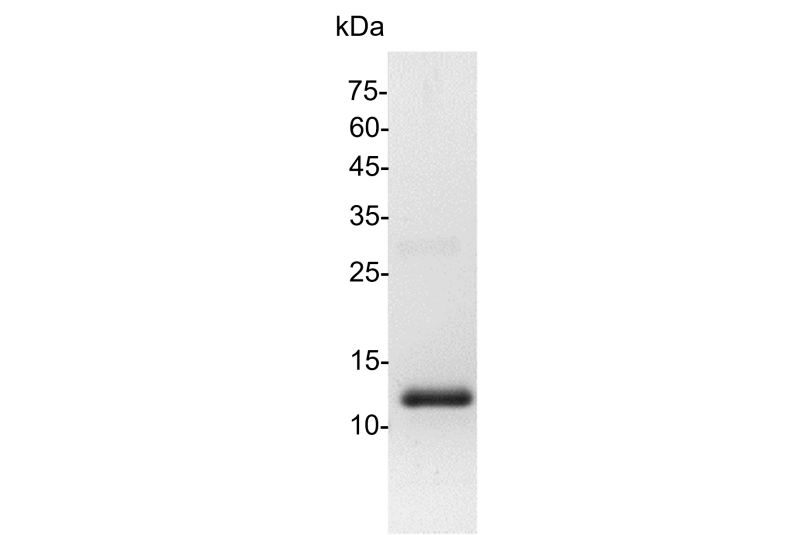
>98% as determined by SDS-PAGE analysis.
Form
Lyophilized
Storage Buffer
Lyophilized from a 0.2 µm filtered solution of PBS, pH 8.0.
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Stability & Storage
This product is stable after storage at:
• -20°C for 12 months in lyophilized state from date of receipt.
• -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Shipping conditions
Blue ice
Background Information
Transforming growth factor beta 1 (TGF beta 1) is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation, and apoptosis. In humans, TGF-β1 is encoded by the TGFB1 gene.
Quality Statement
Croyez GMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP-certified facility.
The processes include:
● Animal-free reagent and laboratory
● Manufactured and tested under GMP guideline
● Testing and traceability of raw material
● Records of the maintenance and equipment calibration
● Personnel training records
● Batch-to-batch consistency
● Documentation of QA control and process changes
● Manufactured and tested under an ISO 13485:2016 certified quality management system
● Stability monitor of product shelf-life
Quality Assurance
At Croyez, we are committed to providing our valued customers with detailed product analytics to assist in their research endeavors. Our Certificate of Analysis includes the following lot-specific details:
● SDS-PAGE analysis and endotoxin level evaluation conducted on bulk QC lots.
● Lot-specific bioassay results ensuring compliance with established parameters, including microbial testing meeting USP <71> standards.
● Mycoplasma detection via PCR analysis.
We believe in transparency and strive to empower our customers with comprehensive information to aid in their decision-making process.
Downloads
→ Materials For iPSC differentiation
→ [ Recombinant Protein ] The Key Material of Cell Therapies: GMP-Grade Recombinant Proteins
Related Articles
→ [ Recombinant Protein ] Exploring the Diversity: Conventional T Cells Dance to Different Tunes of Immunity!
→ [ Recombinant Protein ] The Impact of Cytokines on NK Cells: Shaping Their Activity and Function
→ [ Recombinant Protein ] A spy: IL-1β induces acute inflammation to kill the cancer cells.
→ [ Recombinant Protein ] Exploring the Diversity: Conventional T Cells Dance to Different Tunes of Immunity!
→ [ Recombinant Protein ] A micromanager: granulocyte-macrophage colony-stimulating factor (GM-CSF) from growth factor to immune modulator
Related Product
→ TGF beta 1 (Transforming growth factor β1), Human
Average Rating: 0 (0 Reviews )